代表性科研论文
https://orcid.org/0000-0002-6710-3798
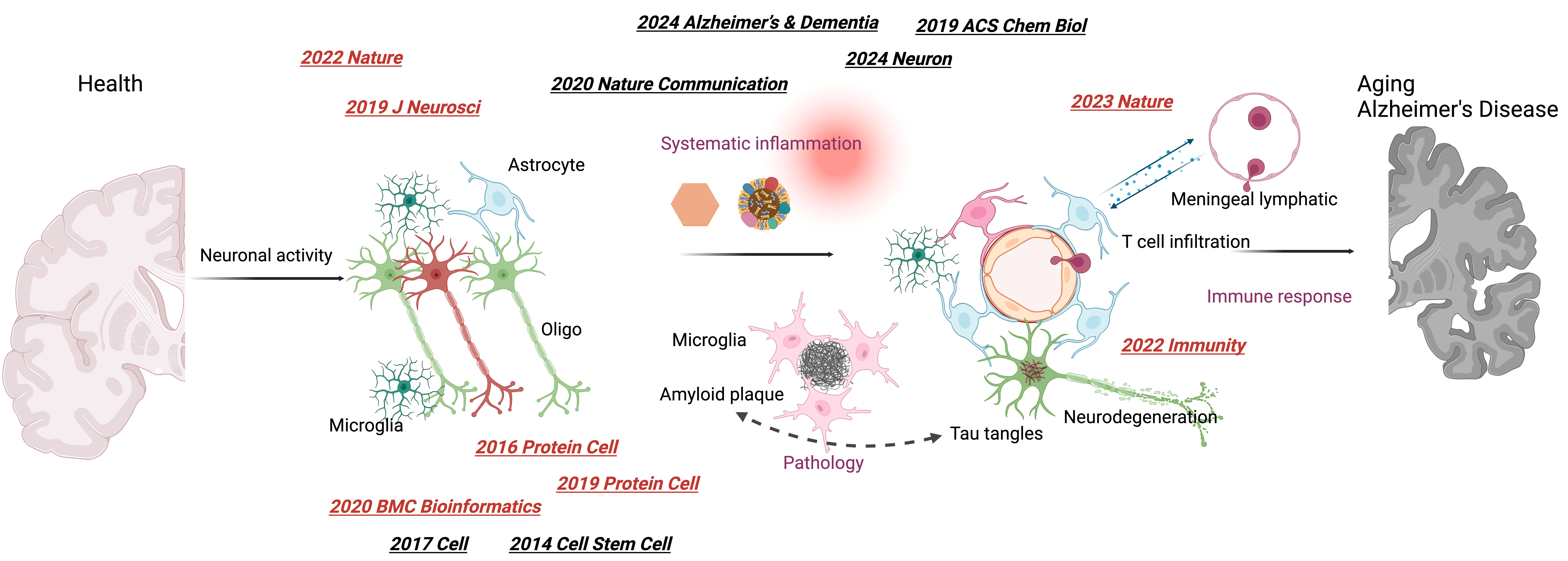
I-神经免疫在神经退行性疾病中的作用机制
陈晓颖博士和团队通过系统分析Aβ和Tau两种AD模型,结合大量AD病人不同疾病阶段脑样本,首次发现在神经退行性疾病的发生发展中,大脑存在固有免疫和获得性免疫相互促进的异常免疫应答,打破这一异常免疫应答中的任何一个环节将有望能延缓或逆转AD晚期病理改变 。
Carling G, Fan L, Foxe N, Norman K, Wong M, Zhu D, Corona C, Razzoli A, Yu F, Yarahmady A, Ye P, Chen H, Huang Y, Amin S, Sereda R, Lopez-lee C, Zacharioudakis E, Chen X, Xu J, Cheng F, Gavathiotis E, Cuervo A, Holtzman D, Mok S, Sinha S, Sidoli S, Ratan R, Luo W, Gong S, Gan L. Alzheimer’s disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model. 2024. Neuron. 112. 1-20, PMID: 38328219
AAIC, Kloske C, Tansey M, Wilcock D (Chen X, leading the study of Microglia-mediated T-cell infiltration and reactivity) Advancements in Immunity and Dementia Research: Highlights from the 2023 AAIC Advancements: Immunity Conference. 2024. Alzheimer’s and Dementia. PMID: 39692624
AAIC, Kloske C, Bu G, Goate A, Holtzman D (Chen X, leading the study of APOE and immune response in Alzheimer’s Disease) Advancements in APOE and dementia research: Highlights from the 2023 AAIC Advancements: APOE conference. 2024. Alzheimer’s and Dementia. 20, 5815-6664, PMID: 39031528
Chen X, Firulyova M, Manis M, Herz J, Smirnov I, Aladyeva E, Wang C, Bao X, Finn M, Hu H, Shchukina I, Kim M, Yuede C, Kipnis J, Artyomov M, Ulrich J, Holtzman D. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. 2023. Nature. 615, 668-677, PMID: 36890231
Chen X, Holtzman D. Emerging roles of innate and adaptive immunity in Alzheimer’s Disease. 2022. Immunity. 55, 2236-2254, PMID: 36351425
II-遗传与表观遗传在学习记忆中的作用机制
哺乳动物中枢神经系统是非常复杂的,有上亿的神经元。在学习记忆中,什么样的神经元才能参与这一活动?神经元彼此之间是怎样协同互作的?基因和表观层面又是如何精准调控的?功能和种类相近的神经元往往聚集形成核团,解剖学对这些核团进行了定义,并描绘了其上下游投射环路。陈晓颖博士和团队建立了高效、稳定的能有效捕获特定行为反应性神经元,并解析反应性神经元可塑改变的方法,解析了参与运动学习记忆的百年来只知环路结构,未知分子图谱的复杂浦肯野神经元类型,揭示了浦肯野神经元的分型、运动学习反应性神经元的分子特征、可塑性分子改变以及学习记忆的关键信号通路, 回答了基因和表观对神经元参与学习记忆精准调控的核心脑科学问题。
Chen X, Du Y, Broussard J, Kislin M, Yuede C, Zhang S, Dietmann S, Gabel H, Zhao G, Wang S, Zhang X, Bonni A. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. 2022. Nature. 605, 722-727, PMID: 35545673
Chen X, Zhang B, Wang T, Bonni A, Zhao G. Robust principal component analysis for accurate outlier detection in RNA-Seq data. 2020. BMC Bioinformatics. 21, 269. PMID: 32600248
Chen X, Chanda A, Ikeuchi Y, Zhang X, Goodman JV, Reddy NC, Majidi SP, Wu DY, Smith
SE, Godec A, Oldenborg A, Gabel HW, Zhao G, Bonni S, Bonni A. Transcriptional regulator SnoN promotes proliferation of cerebellar granule neuron precursors in the postnatal mouse brain. 2019. J Neurosci. 39, 44-62. PMID: 30425119
Smith SE, Chen X, Brier L, Bumstead J, Rensing N, Epstein A, Oldenborg A, Crowley J, Bice
A, Dikranian K, Ippolito J, Haigis M, Papouin T, Zhao G, Wong M, Culver JP, Bonni A.
Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway. 2020. Nature Communication. 11, 6164. PMID: 33268780
Krishnan N, Chen X, Donnelly-Roberts D, Mohler E.G, Holtzman D. M, Gopalakrishnan, S. M. Small molecule phenotypic screen identifies novel regulators of LDLR expression. 2020. ACS Chem Biol.15, 3262-3274. PMID: 33270420
III-免疫和代谢微环境调控神经发育和神经元成熟
人类神经系统的功能是通过不同类型的神经元所形成的精准神经环路所执行的。由于人神经系统的进化属性,人神经发育必然有别于其它模式动物,领域内迫切需要建立一套完善的研究体系用于人神经发育的相关研究,解析神经元细胞命运决定和神经元功能成熟等核心发育事件的调控机制。基于人神经发育原理,陈晓颖博士建立人多能干细胞(hPSCs)通过神经诱导(Neural induction)、区域化(Neural patterning)、神经元生成(Neurogenesis)等阶段的定向分化体系。为了揭示调控人神经元电生理成熟的分子机制,建立了偶联神经元膜片钳记录与同一神经元单细胞转录组关联分析(Patch-seq)方法,通过膜片钳记录区分不成熟、成熟中和成熟各亚型人神经元,然后通过记录电极吸取神经元内容物进行单细胞转录组深度测序。研究发现蛋白质泛素化修饰,神经免疫调控和细胞代谢等通路在神经元电生理成熟过程中发挥关键调控作用,揭示了免疫和代谢微环境对神经发育的关键调控作用。
Chen X*, Han X, Blanchi B, Guan W, Ge W, Yu YC*, Sun YE*. Graded and pan-neural disease phenotypes of Rett Syndrome linked with dosage of functional MeCP2. 2020. Protein Cell. 12, 639-652. PMID: 32851591 (co-corresponding author)
Chen X, Zhang K, Zhou L, Gao X, Wang J, Yao Y, He F, Luo Y, Yu Y, Li S, Cheng L, Sun YE.
Coupled electrophysiological recording and single cell transcriptome analyses revealed molecular mechanisms underlying neuronal maturation. 2016. Protein Cell. 7, 175-186. PMID: 26883038 (Cover research)
Chen Y, Yu J, Niu Y, Qin D, Liu H, Li G, Hu Y, Wang J, Lu Y, Kang Y, Jiang Y, Wu K, Li S, Wei J, He J, Wang J, Liu X, Luo Y, Si C, Bai R, Zhang K, Liu J, Huang S, Chen Z, Wang S, Chen X, Bao X, Zhang Q, Li F, Geng R, Liang A, Shen D, Jiang T, Hu X, Ma Y, Ji W, Sun YE. Modeling Rett Syndrome Using TALEN-Edited MECP2 Mutant Cynomolgus Monkeys.
2017. Cell. 169, 945-955. PMID: 28525759
Liu H, Chen Y, Niu Y, Zhang K, Kang Y, Ge W, Liu X, Zhao E, Wang C, Lin S, Jing B, Si C, Lin Q, Chen X, Lin H, Pu X, Wang Y, Qin B, Wang F, Wang H, Si W, Zhou J, Tan T, Li T, Ji S, Xue Z, Luo Y, Cheng L, Zhou Q, Li S, Sun YE, Ji W. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. 2014. Cell Stem Cell. 14, 323-328. PMID: 24529597
科研成果总结
-Ten years scientific journey in filling the gap of neuron-immune interactions